Explain the Relationship Between Electronegativity Difference and Polarity
Polar bonds and polar molecules. The difference in electronegativity between two atoms determines how polar a bond will be.
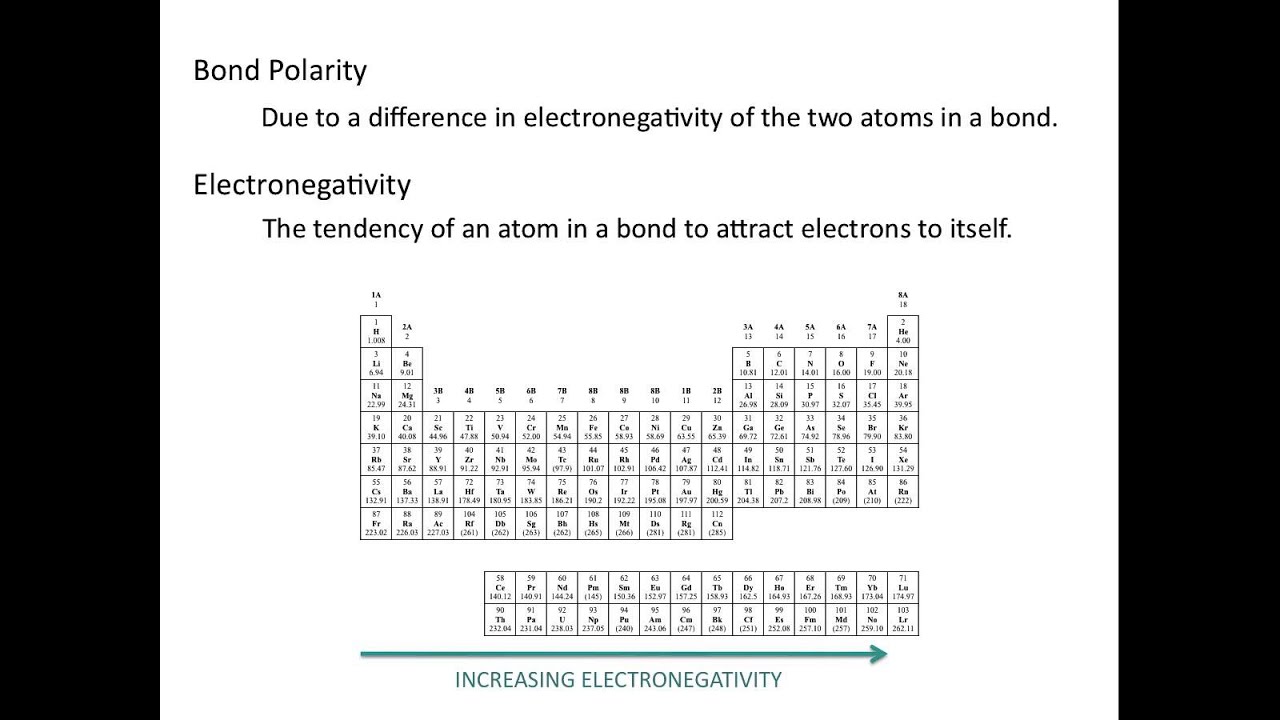
What Is The Relationship Between Electronegativity And The Polarity Of A Chemical Bond Lisbdnet Com
The degree to which an atom attracts electrons in a chemical bond is described by electronegativity.

. If the electronegativity difference is less than 05 the bond is nonpolar. This creates a partially negative charge at the oxygen and a partially positive charge at the hydrogen. Dielectric constant is the ability of a certain material to store under the influence of an electric field electrical potential energy.
To determine the polarity of a covalent bond using numerical means the difference between the electronegativity of the atoms is taken. When is a molecule polar. Basically if a molecule is polar it means that one side of the molecule has a higher affinity for electrons and will attract them more strongly leading to a more positively charged side and a more negatively charged side.
2 shared pairs of electrons. Explain the relationship between electronegativity and polarity. Up to 24 cash back If two different atoms are bonded they form a polar bond as there is an electronegativity difference and the valence electrons spend more time around the atom with the higher electronegativity value that atom becomes slightly negative The atom that the valence electrons spend less time around becomes slightly positive.
The difference in the electronegativity of two atoms determines their bond type. What is the difference between polar and nonpolar molecules in terms of their electronegativity difference. Bond polarity arises due to the differences in electronegativity values of atoms.
If the electronegativity difference is over. If the result is between 04. In polar molecules the difference in electronegativity among the bonding atoms results in the polarity of bonds on the other side.
Change the bond angle to see how shape affects polarity. In non-polar molecules the difference of. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity.
1 shared pair of electrons. If the two atoms in the bond have no electronegativity difference then a pure covalent bond will form. Molecular polarity is mainly dependent on the geometry of the molecule.
If the electronegativity difference is more than 17 the bond will have an ionic character. A large electronegativity difference leads to an ionic bond. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons while polarity is the property of having poles or being polar.
On the Pauling scale if the result is less than 04 the bond is generally nonpolar covalent. 3 Shared pair of electrons. The polarity of a chemical.
HF is polar because both the bonding atoms are not same thus there is an electronegativity difference between the atoms which results in the development of poles in the molecules since the electron pair will not lie exactly in the middle of the two atoms because F being more electronegative attract the pair of electron towards itself thus this part of molecule. Compare and contrast single double and triple bonds. Fluorine the most electronegative.
Difference Between Dielectric Constant and Polarity What is Dielectric Constant. If there is a slight difference a polar covalent bond will form. Dielectric permeability is a physical property describing how the electric field influences the dielectric environment and how it.
Subsequently one may also ask how do you explain polarity. For a bond to be polar the electronegativity difference between the two elements needs to be higher than 05. Electronegativity is the key factor that differentiates between polar and nonpolar bonds.
Click to display the graph showing the relationship between polarity P and electronegativity difference ΔEN Using the idea of electronegativity difference for predicting polarity of bonds and considering the shape of the following molecules match the compounds in the order of strongest to weakest expected polarity. So if there is a larger difference of electronegativity between 2. 4 then generally that means that its polar.
Electronegativity is also important in determining the nature of bonds. Moreover if the electronegativity difference between the two is high then an ionic bond will be the result. If the difference in electronegativity is greater than 17 the character of the bond will be ionic.
So the key difference between electronegativity and polarity is that the electronegativity is the tendency of an atom to attract the electrons in a bond towards it whereas polarity is the separation of the charges. However the main difference between bond polarity and molecular polarity is that bond polarity explains the polarity of a covalent bond whereas molecular polarity explains the polarity of a covalent molecule. It is also called permittivity.
4 rows Electrons in a polar covalent bond are shifted toward the more electronegative atom. A small electronegativity difference leads to a polar covalent bond. No electronegativity difference between two atoms leads to a pure non-polar covalent bond.
In a simple molecule like HCl if the bond is polar so also is the whole molecule. In a diatomic molecule with two identical atoms there is no difference in electronegativity so the bond is nonpolar covalent. If the electronegativity difference is between 04 and 17 the bond will have a polar covalent character.
The polarity of a bond is defined by the unequal sharing of the electrons between 2 molecules. Electronegativity is the measure of an atom to attract electrons for bonding. If the electronegativity difference between the two atoms is above 17 then ionic bond is formed and if the difference is below 17 then covalent bond is formed.
Change the electronegativity of atoms in a molecule to see how it affects polarity. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent. When a covalent bond sharing is created between two atoms oxygen and hydrogen the atom oxygen with the greater electronegativity gets the BIGGER share of the electron pair.
See how the molecule behaves in an electric field. Taking into account their definitions the relationship between the bond dipoles and molecule dipole is that the polarity is characteristic of each bond and is due to the difference of electronegativity between the bonded atoms while the sum of all the polarities of the bonds in the molecule results in the polarity of the molecule. The greater the electronegativity difference the more polar the bond.

Bond Type Using Electronegativity Youtube
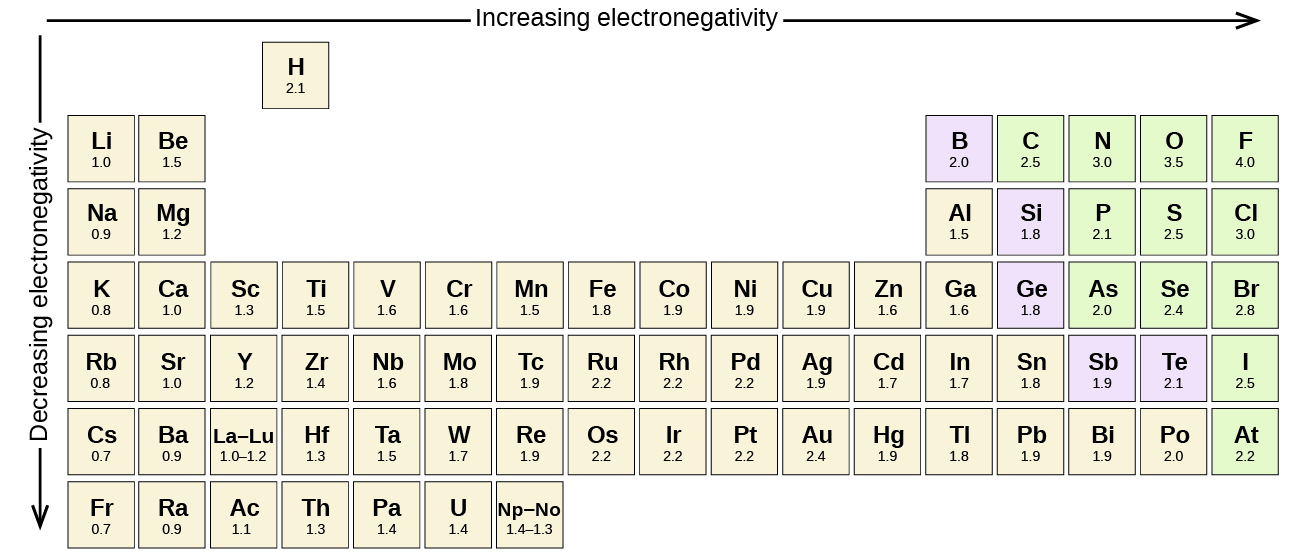
6 1 Electronegativity And Polarity Chemistry Libretexts

6 4 Polarity Of Molecules Introductory Chemistry

Video And Practice Quiz Polarity In Bonds Due To Difference In Electronegativity Chemtopper Com
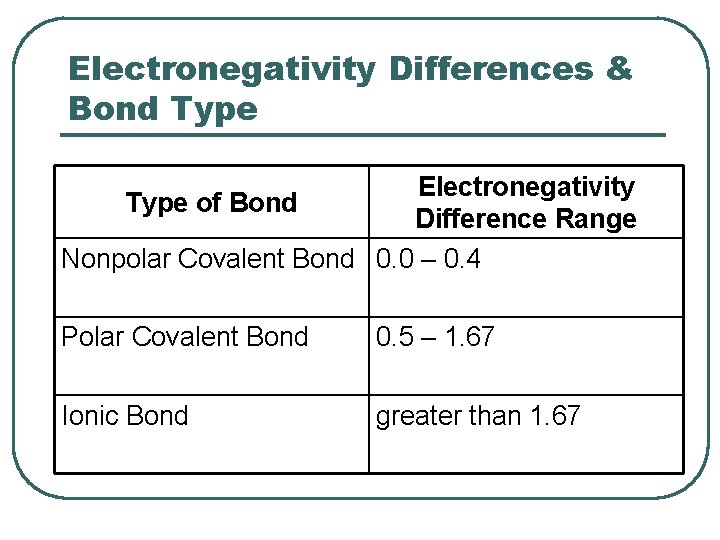
Chemical Bonding Polarity Of Compounds Bond Polarity Nonpolar
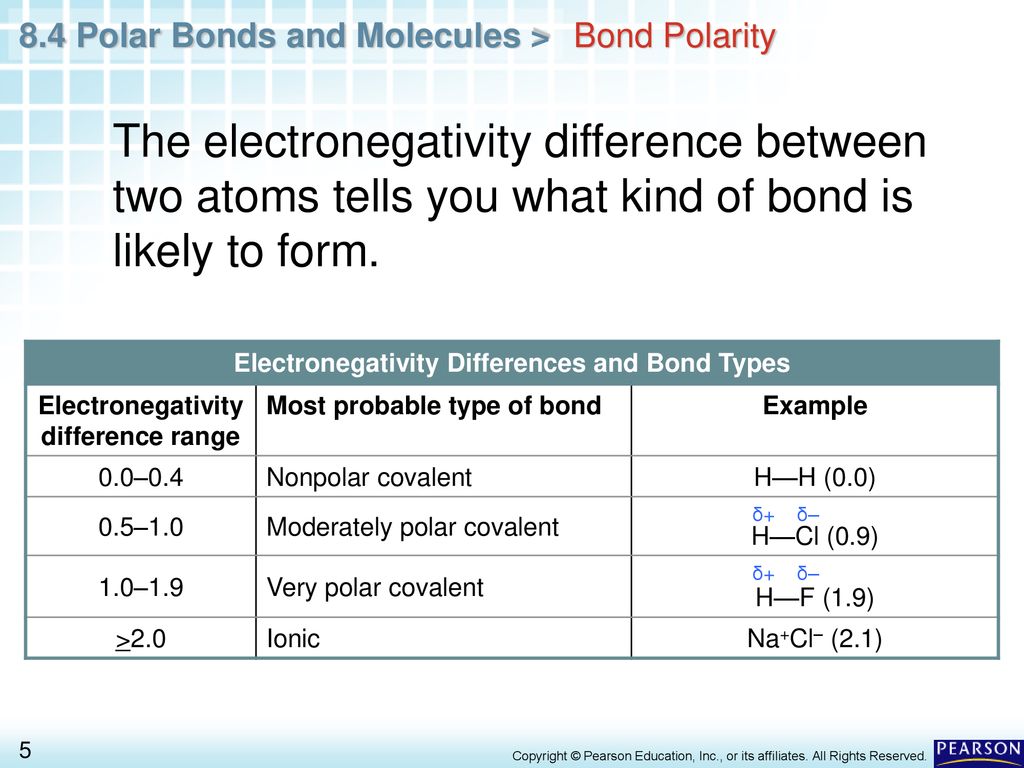
Chapter 8 Covalent Bonding 8 4 Polar Bonds And Molecules Ppt Download

5 3 Polarity And Intermolecular Forces Chemistry Libretexts

Difference Between Electronegativity And Polarity Compare The Difference Between Similar Terms

Electronegativity And Polarity Ppt Download

Difference Between Electronegativity And Polarity Compare The Difference Between Similar Terms
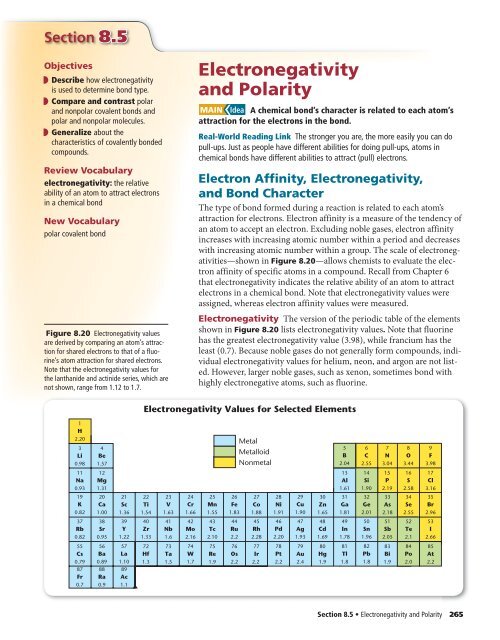
Electronegativity And Polarity Mcgraw Hill Higher Education
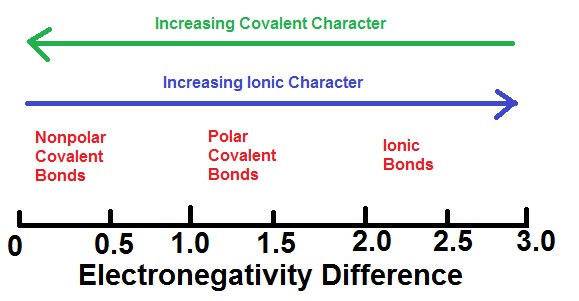
M8q1 Bonding And Electronegativity Chem 103 104 Resource Book
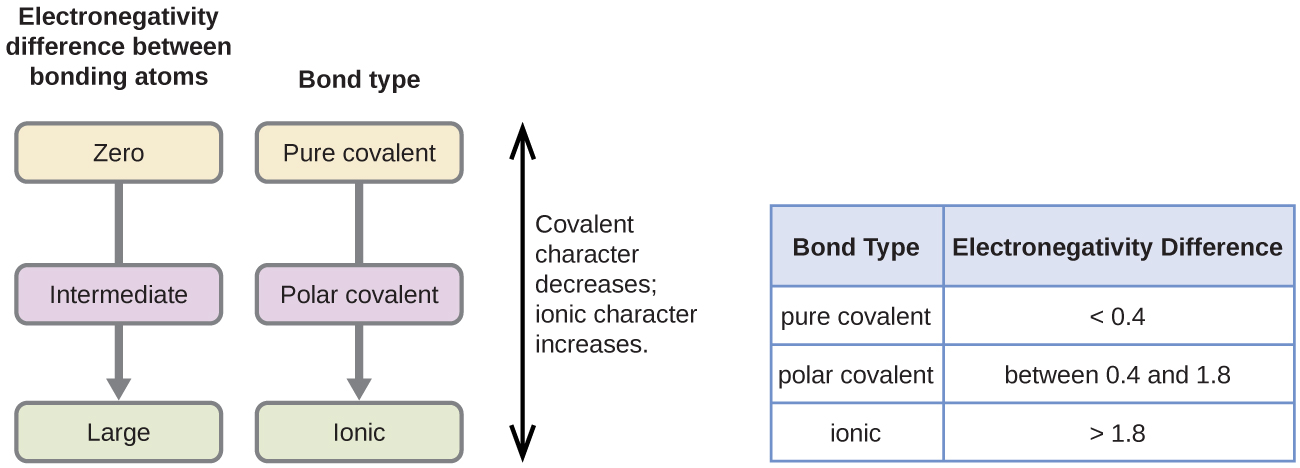
4 8 Polar Covalent Bonds And Electronegativity Chemistry Libretexts

Polarity It Exists In Two Forms Bond Polarity We Looking At The Difference In Electronegativies Between Atoms To Determine How They Share Their Electrons Ppt Download
12 2 Electronegativity Chemistrysaanguyen

Videoquiz Definition Electronegativity Polar Dipole Electron Affinity


Comments
Post a Comment